
CLDN18.2 Ring Study in Germany: Methods1
A total of 10 consensus-scored and validated gastric cancer cases (6 CLDN18.2-positive and 4 CLDN18.2-negative) were selected from the IPT and sent to participating laboratories for the OPT
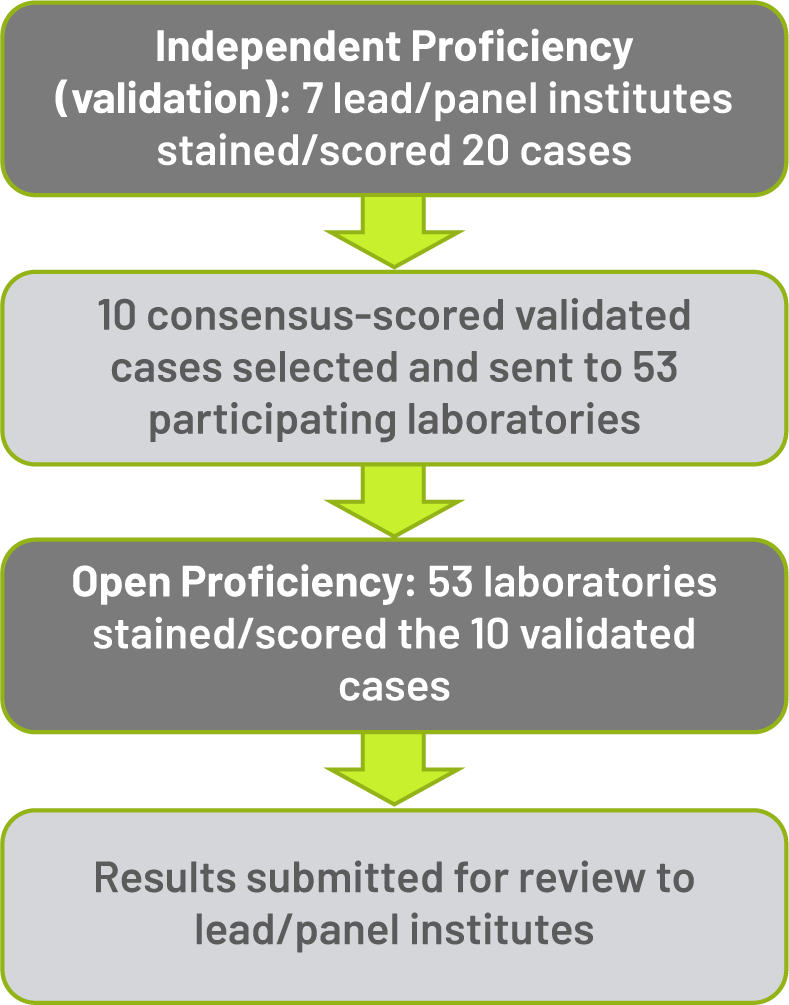
INDEPENDENT PROFICIENCY TEST: Of the 20 cases, 18 (90%) were evaluated consistently among the 7 institutions
OPEN PROFICIENCY TEST: The 10 selected cases were tested by 53 institutions. 42 institutions participated successfully (79.2%) by correctly evaluating CLDN18.2 status in ≥ 90% of cases
Main reasons for discordance were related to factors in staining protocols. Of the 53 institutes:
| Antibody (Manufacturer) | Total Number of Laboratories Using Antibody | Successful Categorization (Passing Rate, %) | Problem Analysis | ||
|---|---|---|---|---|---|
| Staining | Interpretation | Staining and Interpretation | |||
| 43-14A (Abcam) | 2 | 2 (100) | - | - | - |
| Polyclonal rabbit (Invitrogen/Thermo Fisher) | 2 | 2 (100) | - | - | - |
| VENTANA CLDN18 (43-14A) Assay (Roche Diagnostics) | 28 | 27 (96) | 1 | 2 | - |
| PathPlus™ CLDN18 (LSBio) | 3 | 2 (67) | - | 1 | - |
| ZR451 (Zeta/Zytomed)a | 9 | 5 (56) | 7 | - | 1 |
| EPR19202 (Abcam) | 5 | 0 (0) | 5 | - | - |
a Participants who participated successfully with this clone submitted suboptimal stains.
ZR451
EPR19202
In a global study assessing the reproducibility and comparability of three CLDN18 antibodies and IHC staining platforms across a cohort of 27 global laboratories2,*,†:
*Antibodies in the study comprised the VENTANA CLDN18 (43-14A) IVD Assay from Roche Tissue Diagnostics, the PathPlus™ CLDN18 Antibody from LSBio, and the Claudin-18 Antibody from Novus Biologicals. Platforms comprised BenchMark ULTRA, Dako Autostainer, and Leica Bond.2
†Consensus reference scores from all antibodies for each sample were determined by central pathology review. CLDN18.2 positivity was defined with a threshold of ≥75% of tumour cells expressing membranous CLDN18 with moderate-to-strong (≥2+) staining intensity. Accordingly, participating pathologists were required to submit a binary positive/negative call as well as an estimation of the percent of cells stained. Laboratory-submitted IHC scores were compared to the reference consensus score and considered discordant if the positive/negative binary result differed. Statistical analysis was performed for comparison, and an acceptance criteria of 85% (≥0.85) was applied.2
CLDN18, claudin 18; CLDN18.1, claudin 18 isoform 1; CLDN18.2, claudin 18 isoform 2; IHC, immunohistochemistry; IPT, independent proficiency test; IVD, in vitro diagnostic; LDT, laboratory developed test; OPT, open proficiency test.
References: 1. Röcken C, Höhn AK, Neumann J, et al. Concordance of laboratory assays for claudin 18.2 in gastric cancer tissue samples: independent proficiency testing and a descriptive non-interventional study. Virchows Arch 2025 Jun 28. 2. Jasani B, Schildhaus HU, Taniere P, et al. Global ring study determining reproducibility and comparability of CLDN18 testing assays in gastric cancer. Poster presented at: ESMO Targeted Anticancer Therapies Congress; March 6-8, 2023; Paris, France.